All-stock transaction maintains AC Immune's strong cash position
USD 25 million private placement led by premier investor Athos Service GmbH
Transactions expand and accelerate AC Immune's therapeutic development in Parkinson's disease
AC Immune to immediately launch clinical development of acquired vaccine into an adaptive, biomarker-based Phase 2 study in Parkinson's disease
Conference call scheduled today at 8:30 am ET / 2:30 pm CET
LAUSANNE, Switzerland, July 27, 2021 (GLOBE NEWSWIRE) -- AC Immune SA (NASDAQ: ACIU), a clinical-stage biopharmaceutical company pioneering precision medicine for neurodegenerative diseases, today announced that it is acquiring Affiris' portfolio of therapeutics targeting alpha-synuclein (a-syn), notably Affiris PD01, a clinically-validated active vaccine candidate for the treatment of Parkinson's disease, as well as cash. The all-stock asset acquisition is valued at USD 58.7 million. AC Immune's cash position, as well as the Company's investor base, is also being strengthened by a total of USD 30 million in gross proceeds stemming from the asset acquisition and a parallel financing that are adding Athos Service GmbH (Strüngmann family office), First Capital Partner GmbH (Egger Family Office)1 and MIG Fonds2, the lead investors in Covid-19 vaccine innovator BioNTech SE, as new AC Immune shareholders.
Under the terms of the agreement, AC Immune is acquiring all of Affiris' assets and underlying intellectual property related to active vaccine candidates targeting a-syn and USD 5 million in cash for 7.1 million shares based on a price of USD 8.26 per common share. This share price represents a 10.7% premium compared to the closing price of AC Immune shares as of July 23, 2021.
Concurrent with the asset acquisition, AC Immune announced a private placement transaction with a select group of investors led by Athos Service GmbH with additional participation from First Capital Partner GmbH. Gross proceeds from the private placement will be USD 25 million, based on the same financial terms as for the acquisition of the therapeutic anti-a-syn portfolio. Another lead BioNTech and Affiris investor, MIGoFonds, is also becoming a new AC Immune shareholder via its prior ownership of the anti-a-syn assets acquired. In total, AC Immune is issuing 10.1 million shares in conjunction with the asset acquisition and related financing, in exchange for the aforementioned anti-a-syn assets valued at USD 53.7 million and USDo30 million in cash.
With the support of their new premier investors, who are highly experienced in active vaccination, AC Immune will leverage its own extensive expertise in vaccine development and neurodegenerative diseases to immediately launch clinical development of ACI-7104, the optimized formulation of PD01, into an adaptive, biomarker-based Phase 2 study. This trial will evaluate an initial dose-response of the optimized formulation focusing on immunogenicity against a-syn and pathological a-syn species. Additionally, progression of motor and non-motor symptoms of Parkinson's disease will be monitored, together with digital, imaging and fluid biomarkers.
Michael Motschmann, General Partner of MIG Fonds, added: "AC Immune is uniquely positioned to successfully advance the industry-leading anti-a-syn vaccine program developed by Affiris and its investors. The program will benefit from AC Immune's deep expertise in precision medicine and active vaccination approaches for neurodegenerative diseases. We are thrilled to be joining their high-quality investor base and believe this transaction will be of great benefit to our shareholders and the millions of patients suffering from neurodegenerative diseases around the world."
Thomas Strüngmann, Co-Founder of Athos Service GmbH, commented: "We have learned from Covid-19 that collaboration can accelerate the development of safe and efficacious vaccines that can be deployed broadly to deliver highly effective protection for very large populations. This is why we strongly endorse this transaction with AC Immune, a global leader in vaccine development in neurodegeneration with an impressive precision medicine pipeline and record of execution. We look forward to working with the Company to potentially translate what has been done for infectious diseases to neurodegenerative diseases like Parkinson's and Alzheimer's."
Prof. Andrea Pfeifer, CEO of AC Immune SA, commented: "We believe very strongly that active vaccination will play an important role in the long-term management and possible prevention of neurodegenerative diseases. By acquiring a clinically validated vaccine program against a-syn, we are advancing AC Immune to the forefront of Parkinson's disease drug development, with a wholly-owned a-syn portfolio that now covers a full spectrum of treatment modalities addressing this well-characterized target."
"We are also proud to welcome three of the foremost investors in vaccine development, the Strüngmann Family's Athos Service, First Capital Partner GmbH and MIG Fonds, to AC Immune. As the lead investors in BioNTech, they have a globally recognized track record of identifying and supporting successful vaccine technologies. Their support of Affiris and now AC Immune serves as an endorsement of an active immunization approach for Parkinson's disease, and provides key external validation for AC Immune's unmatched expertise in the development of vaccines for neurodegenerative diseases."
Parkinson's disease is causally linked to pathological aggregation and deposition of a-syn, a drug target that has been validated in clinical studies using monoclonal antibodies. Affiris PD01 is a vaccine designed to generate a target-specific antibody response against pathological oligomeric a-syn. Randomized Phase 1 results published in The Lancet Neurology showed that PD01 generated substantial, long-lasting and boostable antibody responses against pathological forms of a-syn that were accompanied by target engagement and signals of clinical efficacy. These data also showed that PD01 had a favorable safety and tolerability profile. This anti-a-syn vaccine complements AC Immune's existing portfolio of clinical and preclinical candidates addressing a-syn, which includes an anti-a-syn antibody, a small molecule aggregation inhibitor, and a first-in-class diagnostic imaging agent, which together may enable a precision medicine approach to treating Parkinson's disease and other alpha-synucleinopathies. With this acquisition, AC Immune becomes a leader in the development of therapeutics and diagnostics for Parkinson's disease.
AC Immune's expertise in active vaccine development is based on its SupraAntigen®-V vaccine platform, which has been shown to generate powerful, target-specific, polyclonal antibody responses that are in the range of therapeutic monoclonal antibodies. Earlier this year, the Company reported highly encouraging readouts from its ongoing Alzheimer's disease vaccine programs targeting phospho-Tau and pathological amyloid beta (Abeta) including the most toxic oligomeric and pyroglutamate Abeta species. With the recent accelerated approval of the passive immunotherapy Aduhelm™ based on Abeta biomarker data and encouraging clinical results for the anti-Abeta antibody donanemab that demonstrate the potential benefit of targeting pyroglutamate Abeta species, AC Immune is positioned to greatly accelerate the potential time-to-market for its optimized Abeta vaccine. AC Immune is leveraging its extensive vaccine expertise to enrich and accelerate the development of ACI-7104 for Parkinson's disease and other a-synucleinopathies.
The acquisition is subject to customary regulatory approval in Austria and expected to complete at the beginning of Q4 2021.
Conference Call Information
AC Immune will hold a conference call today, July 27, 2021, at 8:30 am ET / 2:30 pm CET. Interested participants and investors may access the conference call by dialing:
- 877-407-0792 (U.S.)
- +1 201-689-8263 (International)
- Conference ID: 13721912
A live webcast will be accessible via the Events section of the Company's website. An archive of the webcast will be available for 90 days beginning at approximately 9:30 am ET / 3:30 pm CET, today, July 27, 2021.
About AC Immune SA
AC Immune SA is clinical-stage biopharmaceutical company that aims to become a global leader in precision medicine for neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, and NeuroOrphan indications driven by misfolded proteins. The Company's two clinically validated technology platforms, SupraAntigen® and Morphomer®, fuel its broad and diversified pipeline of first- and best-in-class assets, which currently features ten therapeutic and three diagnostic candidates, six of which are currently in clinical trials. AC Immune has a strong track record of securing strategic partnerships with leading global pharmaceutical companies including Genentech, a member of the Roche Group, Eli Lilly and Company, and Janssen Pharmaceuticals, Inc., resulting in substantial non-dilutive funding to advance its proprietary programs and >$3 billion in potential milestone payments.
SupraAntigen® and Morphomer® are registered trademarks of AC Immune SA in the following territories: AU, CH, EU, JP, and GB. Morphomer® is also a registered trademark of AC Immune SA in CN and NO.
References:
- https://firstcapitalpartner.de/index.html
- https://www.mig-fonds.de/home.html
For further information, please contact:
Forward-looking statements
This press release contains statements that constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are statements other than historical fact and may include statements that address future operating, financial or business performance or AC Immune's strategies or expectations. In some cases, you can identify these statements by forward-looking words such as "may," "might," "will," "should," "expects," "plans," "anticipates," "believes," "estimates," "predicts," "projects," "potential," "outlook" or "continue," and other comparable terminology. Forward-looking statements are based on management's current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include those described under the captions "Item 3. Key Information – Risk Factors" and "Item 5. Operating and Financial Review and Prospects" in AC Immune's Annual Report on Form 20-F and other filings with the Securities and Exchange Commission. These include: the impact of Covid-19 on our business, suppliers, patients and employees and any other impact of Covid-19. Forward-looking statements speak only as of the date they are made, and AC Immune does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law. All forward-looking statements are qualified in their entirety by this cautionary statement.
Figures accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/5242c349-2642-4a4f-8deb-0b8bf5680015
https://www.globenewswire.com/NewsRoom/AttachmentNg/932e5e0e-7391-4d7d-ab76-0843d0b8c7dc

Source: AC Immune SA
 |
All stock acquisition & PIPE maintains and enhances AC Immune's strong cash position. |
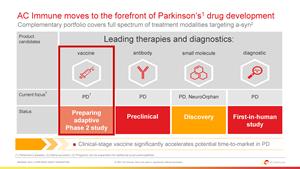 |
ACIU moves to the forefront of Parkinson's drug development |

