AC Immune Reports First Live Images of Alpha-Synuclein in Human Brain with New PET Tracer for Neurodegenerative Disease at AD/PDTM Conference
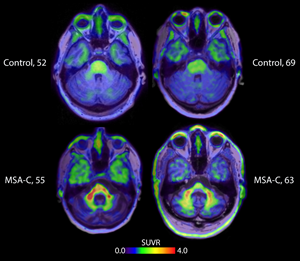
ACI-12589 distinguished multiple system atrophy (MSA) from other a-synucleinopathies and healthy volunteers
A-syn PET tracers may be developed to diagnose a-synuclein pathologies, including Parkinson’s disease, Lewy Body Dementia, MSA and others
LAUSANNE,
The groundbreaking images of a-syn in the human subjects’ brains were presented for the first time today at the AD/PDTM Conference plenary session in
Dr.
Prof. Andrea Pfeifer, CEO of AC Immune SA, commented: “This first clinical validation for an a-syn PET tracer is a transformative step towards achieving our vision for developing precision medicines to treat neurodegenerative diseases. It was made possible by the close collaboration between
The data will be presented in more detail on Friday in two presentations at the peer-reviewed scientific conference1,2. Derived from AC Immune’s Morphomer® technology platform, ACI-12589 showed target engagement in vivo in alpha-synucleinopathies with a pharmacokinetic and safety profile suitable for further development as a human brain PET imaging agent. The trial showed that non-invasive PET brain imaging with ACI-12589 successfully differentiated MSA from other types of a-synucleinopathies, like Parkinson’s disease (PD) and Lewy Body Dementia (LBD), as well as from healthy volunteers.
Specifically, the ACI-12589 PET tracer data showed enhanced contrast and a-syn target specificity in participants with MSA. Tracer retention was highest in areas affected by MSA disease processes, particularly cerebellar white matter.
a-syn Key Opinion Leader webinar on
In addition to the scientific presentations during AD/PDTM,
To register for the webinar, please click here. The materials from the presentation and a replay of the webinar will be available on the Events Page of AC Immune’s website following its conclusion.
References
1Capotosti F.; Discovery of [18F] ACI-12589, a novel and promising PET-tracer for alpha-synuclein; Oral presentation; ADPD 2022
2Smith R.; Initial scans using [18F] ACI-12589, a novel PET-tracer for alpha-synuclein; Oral presentation; ADPD 2022
About Multiple System Atrophy (MSA)
MSA is a rare, degenerative neurological disorder affecting the body’s involuntary (autonomic) functions, including blood pressure, breathing, bladder function and motor control. MSA causes deterioration and shrinkage (atrophy) of portions of the brain (cerebellum, basal ganglia and brainstem) that regulate internal body functions, digestion and motor control. Under a microscope, the damaged brain tissue of people with MSA shows nerve cells (neurons) that contain an abnormally high amount of pathological a-syn protein. MSA is difficult to diagnose and is often confused with PD (about 1 in 40 PD patients has MSA), and, currently, many MSA patients never receive a proper diagnosis. While there is overlap, therapeutic treatment strategies are different in MSA and PD; so, achieving a correct diagnosis is very important.
About
SupraAntigen® is a registered trademark of AC Immune SA in the following territories: AU, EU, CH, GB, JP and RU. Morphomer® is a registered trademark of AC Immune SA in CN, CH, GB, JP, and NO.
For further information, please contact:
| Media Relations Phone: +41 21 345 91 34 Email: saoyuth.nidh@acimmune.com |
Investor Relations Phone: +41 21 345 91 91 Email: gary.waanders@acimmune.com |
LaVoieHealthScience Phone: +1 609 516 5761 Email: slewis@lavoiehealthscience.com |
U.S. Investors Phone: +1 212 915 2577 Email: cdavis@lifesciadvisors.com |
Forward looking statements
This press release contains statements that constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are statements other than historical fact and may include statements that address future operating, financial or business performance or AC Immune’s strategies or expectations. In some cases, you can identify these statements by forward-looking words such as “may,” “might,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “outlook” or “continue,” and other comparable terminology. Forward-looking statements are based on management’s current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include those described under the captions “Item 3. Key Information – Risk Factors” and “Item 5. Operating and Financial Review and Prospects” in AC Immune’s Annual Report on Form 20-F and other filings with the
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/10e79976-ac90-44b1-83f3-ad7e65204765

Source: AC Immune SA